The KT and the dinosaurs
The Cretaceous Period (145-65 Myr ago) was a time of dramatic change in the sea and on land, and the modern pattern of ecosystems was set then, and for the next 100 Myr. It may seem odd to consider dinosaurs as part of a ‘modern’ ecosystem, but they were surrounded by new, or expanded, groups such as the flowering plants, social insects, lizards, snakes, and diverse mammals.
In the marine realm, these ecosystem changes have been named collectively the Mesozoic Marine Revolution, or MMR (Vermeij 1977), characterized by the appearance of new groups of planktonic organisms (e.g. coccoliths, foraminifera, dinoflagellates, diatoms) and new predators among crustaceans, teleost fishes and marine reptiles. It has been postulated (Vermeij 1987) that the emergence of such predators selectively favoured the appearance of thicker exoskeletons as a defensive measure in prey groups such as bivalves, gastropods and echinoids.
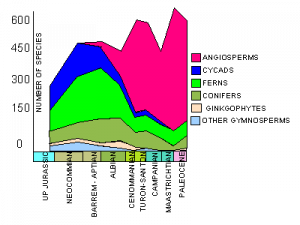 Number of species of different plant groups through the Cretaceous. Note the rise and rise of the angiosperms (coloured pink).
Number of species of different plant groups through the Cretaceous. Note the rise and rise of the angiosperms (coloured pink).
Land-dwelling organisms experienced a similar Cretaceous Terrestrial Revolution, a term we introduced in Lloyd et al. (2008). The abbreviation for the event is KTR, using the standard acronym ‘K’ for the Cretaceous, because the letter ‘C’ is used already for the Carboniferous. The key events during the KTR were the replacement of ferns and gymnosperms by angiosperms (Dilcher 2000). The explosive radiation of angiosperms, from 125-80 Myr ago, provided new evolutionary opportunities for pollinating insects, leaf-eating flies, as well as butterflies and moths, all of which diversified rapidly (Grimaldi 1999).
Key evidence for the KTR and the fact that dinosaurs barely responded came from our supertree of dinosaurs, presented in that 2008 paper.
Among vertebrates, squamates (lizards and snakes), crocodilians, and basal groups of placental mammals and modern birds all underwent major diversifications, although the timing of appearance of modern bird orders and modern mammal orders remains controversial.
 The Early Cretaceous ornithopod Iguanodon enjoys a snack of delicious cycad leaves. Soon, the flowering plants were going to come on the scene, but the mid and late Cretaceous plant- eating dinosaurs do not seem to have adapted their feeding strategies to include this new food source in their diet. Credit: image from Brusatte, S. L. 2008. Dinosaurs. Quercus Publishing, London.
The Early Cretaceous ornithopod Iguanodon enjoys a snack of delicious cycad leaves. Soon, the flowering plants were going to come on the scene, but the mid and late Cretaceous plant- eating dinosaurs do not seem to have adapted their feeding strategies to include this new food source in their diet. Credit: image from Brusatte, S. L. 2008. Dinosaurs. Quercus Publishing, London.
Dinosaur evolution was marked by the appearance of truly spectacular new forms. Giant sauropods, the dominant herbivores of the Jurassic, were joined by new kinds of ornithischians at the beginning of the Cretaceous. Subsequent new waves of diversification at the beginning of the Late Cretaceous (some 100 Myr ago) produced a diverse fauna of hadrosaurs, neoceratopsians, ankylosaurid ankylosaurs and pachycephalosaurs, among herbivores, as well as new carnivorous groups, including the giant carcharodontosaurines and the smaller troodontids, dromaeosaurs and ornithomimosaurs. Qualitatively then, dinosaurs appear to have been part of the KTR.
In fact, only the splitting of the Neoceratopsia and the Euhadrosauria qualify as statistically significant diversification shifts. All those other new groups of herbivorous and carnivorous dinosaurs that appeared at the beginning of the Cretaceous, and in the middle of the period, some 100 Myr ago, count as ‘normal’ evolution, a part of the expected diversificaiton within the clade. The question then is, why did the dinosaurs not evolve explosively in response to the remarkable growth of new plant food?
Did dinosaurs eat angiosperms?
Previous studies have been equivocal about whether dinosaurs fed on angiosperms. The Late Cretaceous expansion of dinosaurian diversity, found especially on the diversification of herbivorous dinosaurs such as hadrosaurs, ceratopsians, and ankylosaurs, might have suggested that these groups, all of which either arose or diversified substantially only after the origin of angiosperms in the mid-Cretaceous, specialized on an angiosperm diet.
Bakker (1978), for example, argued that the ornithopods of the Early Cretaceous fed close to the ground, and so favoured gymnosperms in their diet. Owing to their intense low-level feeding, the only plants that could survive the onslaught were the earliest angiosperms that held their reproductive organs close to the ground. And so, in his words, dinosaurs invented flowers.
This view is disputed and there is actually only limited evidence to demonstrate that Cretaceous dinosaurs fed on angiosperms (Barrett & Willis 2001). The patterns of rises and falls in the diversity of Cretaceous dinosaurs and Cretaceous plants, as well as their palaeogeographic distributions, do not suggest any correlation. Coprolites, fossil faeces, are rare and often cannot be attributed to their producer; Cretaceous examples include some with traces of the angiosperm biomarkers oleananes (a group of chemicals with suppressing effects on insect pests), whereas others contain exclusively gymnosperm material. An Early Cretaceous ankylosaur, Minmi, has been reported (Molnar & Clifford 2000) with remnants of angiosperm fruits in its gut, and some remarkable coprolites from India show that some dinosaurs ate early grasses (Prasad et al. 2005). Fossil occurrences and studies of the teeth and postulated jaw functions of herbivorous dinosaurs suggest that angiosperms were a part of the diet of many dinosaurs, but that gymnosperms were still the major constituent in mostcases (Chin & Gill 1996; Barrett & Willis 2001; Ghosh et al. 2003).
Plant-eating insects and mammals very likely benefited more from the new sources of plant food. Detailed studies of dinosaurian herbivory and plant evolution (Barrett & Willis 2001) had already suggested there was limited evidence that angiosperm diversification drove the Cretaceous diversification of dinosaurs. Our new evidence confirms that the KTR was a key in the origination of modern continental ecosystems, but that the dinosaurs were not a part of it. Hadrosaurs and ceratopsians showed late diversifications, but not enough to save the dinosaur dynasty from its fate.
Impact on mammalian evolution
In subsequent work, Meredith et al. (2011) showed that the KTR had a critical influence on mammalian evolution, in opening up ecospace that promoted interordinal diversification. This suggestion came from close study of a new molecular phylogeny of modern mammalian orders whicxh shows some major splits among orders of placentals and of Euarchontoglires 100 and 83 million years ago respectively, corresponding to the time of rise of angiosperms and new insect groups. Numerical analysis of tree shape shows peaks in diversification shifts at both times, and confirms the earlier suggestion (Lloyd et al. 2008; Benton 2010) that the KTR kick-started important episodes in the evolution of birds and mammals.
References
- Bakker, R.T. 1978. Dinosaur feeding behaviour and the origin of flowering plants. Nature 274, 661-663.
- Barrett, P. M. & Willis, K. J. 2001. Did dinosaurs invent flowers? Dinosaur-angiosperm coevolution revisited. Biological Reviews 76, 411-447.
- Benton, M.J. 2010. The origins of modern biodiversity on land. Philosophical Transactions of the Royal Society, Series B 365, 3667-3679. pdf.
- Chin, K. & Gill, B.D. 1996. Dinosaurs, dung beetles, and conifers: participants in a Cretaceous food web. Palaios11, 280-285.
- Dilcher, D. 2000. Towards a new synthesis: major evolutionary trends in the angiosperm fossil record. Proceedings of the National Academy of Sciences, USA 97, 7030-7036.
- Ghosh, P., Bhattacharya, S. K., Sahni, A., Kar, R. K.,Mohabey, D. M. & Ambwani, K. 2003. Dinosaur coprolites form the Late Cretaceous (Maastrichtian) Lameta Formation of India: isotopic and other markers suggesting a C3 plant diet. Cret. Res. 24, 743-750.
- Grimaldi, D. 1999 The co-radiations of pollinating insects and angiosperms in the Cretaceous. Annals of the Missouri Botanical Garden 86, 373-406.
- Lloyd, G.T., Davis, K.E., Pisani, D., Tarver, J.E., Ruta, M., Sakamoto, M., Hone, D.W.E., Jennings, R., and Benton, M.J. 2008. Dinosaurs and the Cretaceous Terrestrial Revolution. Proceedings of the Royal Society, Series B 275, 2483-2490. pdf
- Meredith, R.W., Janečka, J.E., Gatesy, J., Ryder, O.A., Fisher, C.A., Teeling, E.C., Goodbla, A., Eizirik, E., Simão, T.L., Stadler, T., Rabosky, D.L., Honeycutt, R.L., Flynn, J.J., Ingram, C.M., Steiner, C., Williams, T.L., Robinson, T.J., Burk-Herrick, A., Westerman, M., Ayoub, N.A., Springer, M.S., and Murphy, W.J. 2011. Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science 334, 521-524.
- Molnar, R.E. & Clifford, H.T. 2000. Gut contents of a small ankylosaur. Journal of Vertebrate Paleontology 20, 194-196.
- Prasad, V., Strömberg, C.A.E., Alimohammadian, H. & Sahni, A. 2005. Dinosaur coprolites and the early evolution of grasses and grazers. Science 310, 1177-1180.
- Vermeij, G.J. 1977. The Mesozoic Marine Revolution: evidence from snails, predators and grazers. Paleobiology3, 245-258.
- Vermeij, G.J. 1987. Evolution and escalation. Princeton, NJ: Princeton University Press.

